Description
EVM.CE: HEV IgM – ELISA
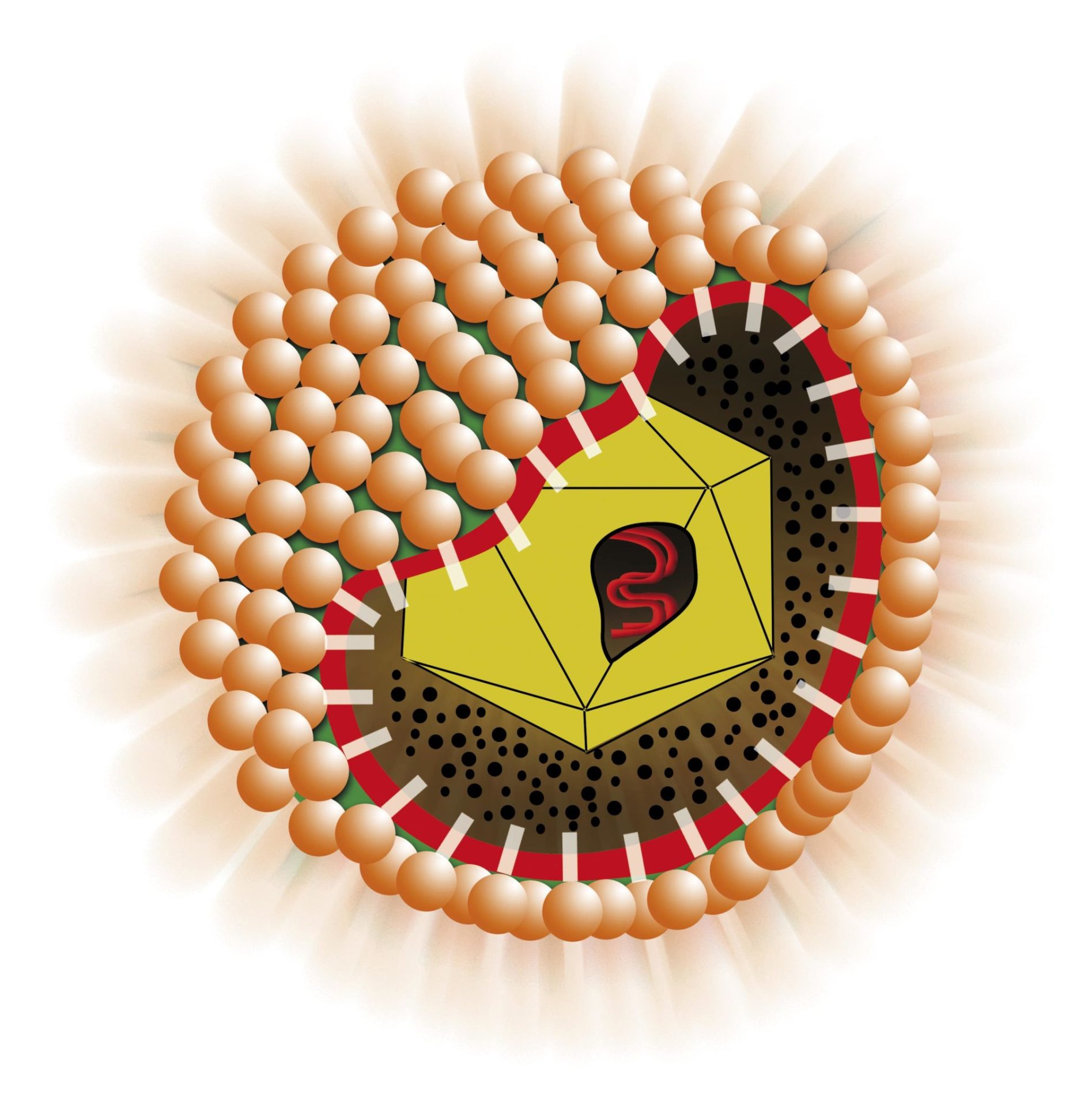
Enzyme ImmunoAssay (ELISA) for the determination of IgM antibodies to Hepatitis E Virus in human plasma and sera. The kit may be used for the follow-up of HEV-infected patients. For “in vitro” diagnostic use only. Microplates are coated with HEV-specific synthetic antigens encoding for conservative and immunodominant determinants derived from Mexican and Burma virus strains. The solid phase is first treated with the diluted sample and anti HEV IgM are captured, if present, by the antigens adsorbed on wells. After washing out all the other components of the sample, in the 2nd incubation bound anti HEV IgM antibodies are detected by the addition of polyclonal specific anti hIgM antibodies, labeled with peroxidase (HRP). The enzyme captured on the solid phase, acting on the substrate/chromogen mixture, generates an optical signal that is proportional to the amount of anti HEV antibodies present in the sample. A cut-off value let optical densities be interpreted into HEV antibody negative and positive results. Neutralization of IgG anti-HEV and Rheumatoid Factor, carried out directly in the well, is performed in the assay in order to block such kind of interferences.
EVG.CE: HEV IgG – ELISA
Third generation Enzyme ImmunoAssay (ELISA) for the qualitative determination of IgG antibodies to Hepatitis E Virus in human plasma and sera. The kit may be used for the screening of blood units and the follow-up of HEV-infected patients. For “in vitro” diagnostic use only. Microplates are coated with HEV-specific synthetic antigens encoding for conservative and immunodominant determinants derived from Mexican and Burma virus strains. The solid phase is first treated with the diluted sample and anti HEV IgG are captured, if present, by the antigens. After washing out all the other components of the sample, in the 2nd incubation bound anti-HEV IgG are detected by the addition of polyclonal specific anti hIgG antibodies, labelled with peroxidase (HRP). The enzyme captured on the solid phase, acting on the substrate/chromogen mixture, generates an optical signal that is proportional to the amount of anti HEV IgG present in the sample. A cut-off value let optical densities be interpreted into anti-HEV IgG negative and positive results.
EVABULTRA.CE.96: HEV Ab (Version ULTRA) – ELISA
Third generation Enzyme ImmunoAssay (ELISA) for the qualitative determination of antibodies to Hepatitis E Virus in human plasma and sera. The kit is for use in screening of blood units and the follow-up of HEV-infected patients. For “in vitro” diagnostic use only.
Microplates are coated with HEV-specific synthetic antigens encoding for conservative and immunodominant determinants derived from Mexican and Burma virus strains.
The solid phase is first treated with the diluted sample and HEV Ab are captured, if present, by the antigens.
2nd incubation bound HEV antibodies, IgG and IgM as well, are detected by the addition of polyclonal specific anti hIgG&M antibodies, labelled with peroxidase (HRP).
The enzyme captured on the solid phase, acting on the substrate/chromogen mixture, generates an optical signal that is proportional to the amount of anti HEV antibodies present in the sample. A cut-off value let optical densities be interpreted into HEV antibody negative and positive results.