Description
BCAB.CE: HBc Ab – ELISA
Competitive Enzyme ImmunoAssay (ELISA) for the determination of antibodies to Hepatitis B core Antigen in human plasma and sera. The kit is for use in screening of blood units and the follow-up of HBV-infected patients.
For “in vitro” diagnostic use only.
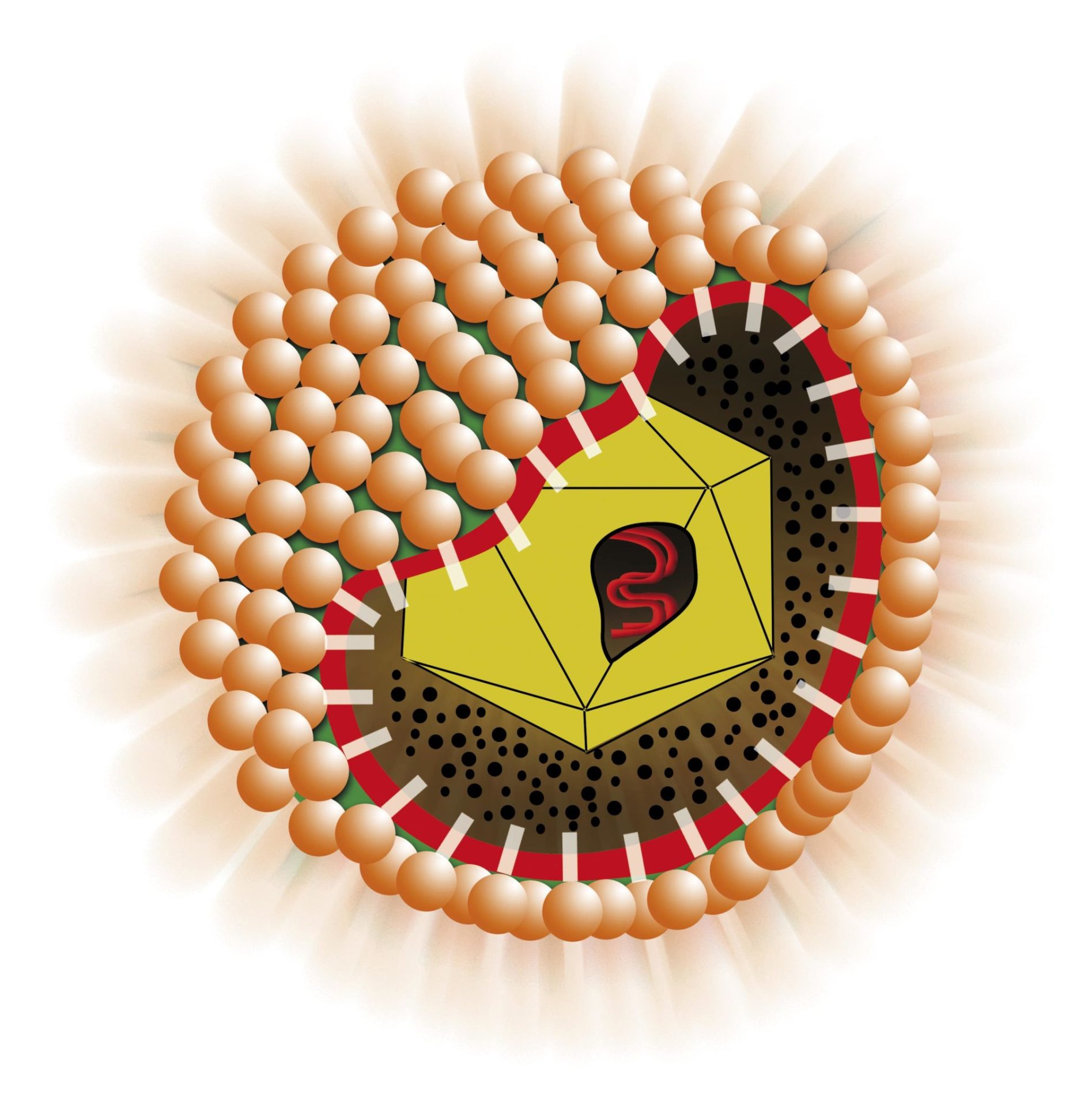
The assay is based on the principle of competition where the antibodies in the sample compete with a monoclonal antibody for a fixed amount of antigen on the solid phase. A purified recombinant HBcAg is coated to the microwells. The patient’s serum/plasma is added to the microwell together with an additive able to block interferences present in the sample. In the second incubation after washing, a monoclonal antibody, conjugated with Horseradish Peroxidase (HRP) and specific for HBcAg is added and binds to the free rec-HBcAg coated on the plastic. After incubation, microwells are washed to remove any unbound conjugate and then the chromogen/substrate is added. In the presence of peroxidase enzyme the colorless substrate is hydrolyzed to a colored end-product.
The color intensity is inversely proportional to the amount of antibodies to HBcAg present in the sample.
HBE.CE: HBe Ag&Ab – ELISA
Enzyme ImmunoAssay (ELISA) for the determination of Hepatitis B Virus “e” Antigen and Antibody in human plasma and sera. The kit is intended for the follow-up of acute infection and of chronic patients under therapy.
For “in vitro” diagnostic use only.
HBeAg, if present in the sample, is captured by a specific monoclonal antibody, in the 1st incubation. In the 2nd incubation, after washing, a tracer, composed of a mix of two specific anti HBeAg monoclonal antibodies, labeled with peroxidase (HRP), is added to the microplate and binds to the captured HBeAg.
SCONF.CE: HBs Ag Confirmation – ELISA
In the screening of blood units for Hepatitis B surface Antigen or HBsAg some false positivity may happen, leading to a misinterpretation of the assay results and a misclassification of the blood unit and the donor. To confirm the positivity of a screened sample or to confirm the presence of an ongoing HBV infection in a hospitalized patient, a confirmatory test has to be run. A simple procedure based on an immunoreaction of neutralization is used in combination with the HBsAg assay.
The device has to be used in combination with the product code SAG1.CE for the determination of HBsAg in human sera and plasma.
BCM.CE: HBc IgM – ELISA
Enzyme ImmunoAssay (ELISA) for both the quantitative and qualitative determination of antibodies to the Surface Antigen of Hepatitis B Virus in human plasma and sera.
For “in vitro” diagnostic use only.
After washing, captured antibodies are detected by an HBsAg, labelled with peroxidase (HRP), that specifically binds the second available binding site of these antibodies. The enzyme specifically bound to wells, by acting on the substrate/chromogen mixture, generates an optical signal that is proportional to the amount of HBsAb in the sample and can be detected by an ELISA reader. The amount of antibodies may be quantitated by means of a standard curve calibrated against the W.H.O reference preparation. Samples are pre treated in the well with an specimen diluent able to block interference present in vaccinated individuals.
SAB.CE: HBs Ab – ELISA
Enzyme ImmunoAssay (ELISA) for both the quantitative and qualitative determination of antibodies to the Surface Antigen of Hepatitis B Virus in human plasma and sera.
For “in vitro” diagnostic use only.
After washing, captured antibodies are detected by an HBsAg, labelled with peroxidase (HRP), that specifically binds the second available binding site of these antibodies. The enzyme specifically bound to wells, by acting on the substrate/chromogen mixture, generates an optical signal that is proportional to the amount of HBsAb in the sample and can be detected by an ELISA reader. The amount of antibodies may be quantitated by means of a standard curve calibrated against the W.H.O reference preparation. Samples are pre treated in the well with an specimen diluent able to block interference present in vaccinated individuals.
SAG1ULTRA.CE: HBs Ag (Version ULTRA) – ELISA
Fourth generation Enzyme Immunoassay (ELISA) for the one-step determination of Hepatitis B surface Antigen or HBsAg in human plasma and sera & for the screening of blood units. Able to detect HBsAg mutants and find application in the follow-up of HBV-infected patients.
For “in vitro” diagnostic use only.