Description
AVM.CE: HAV IgM – ELISA
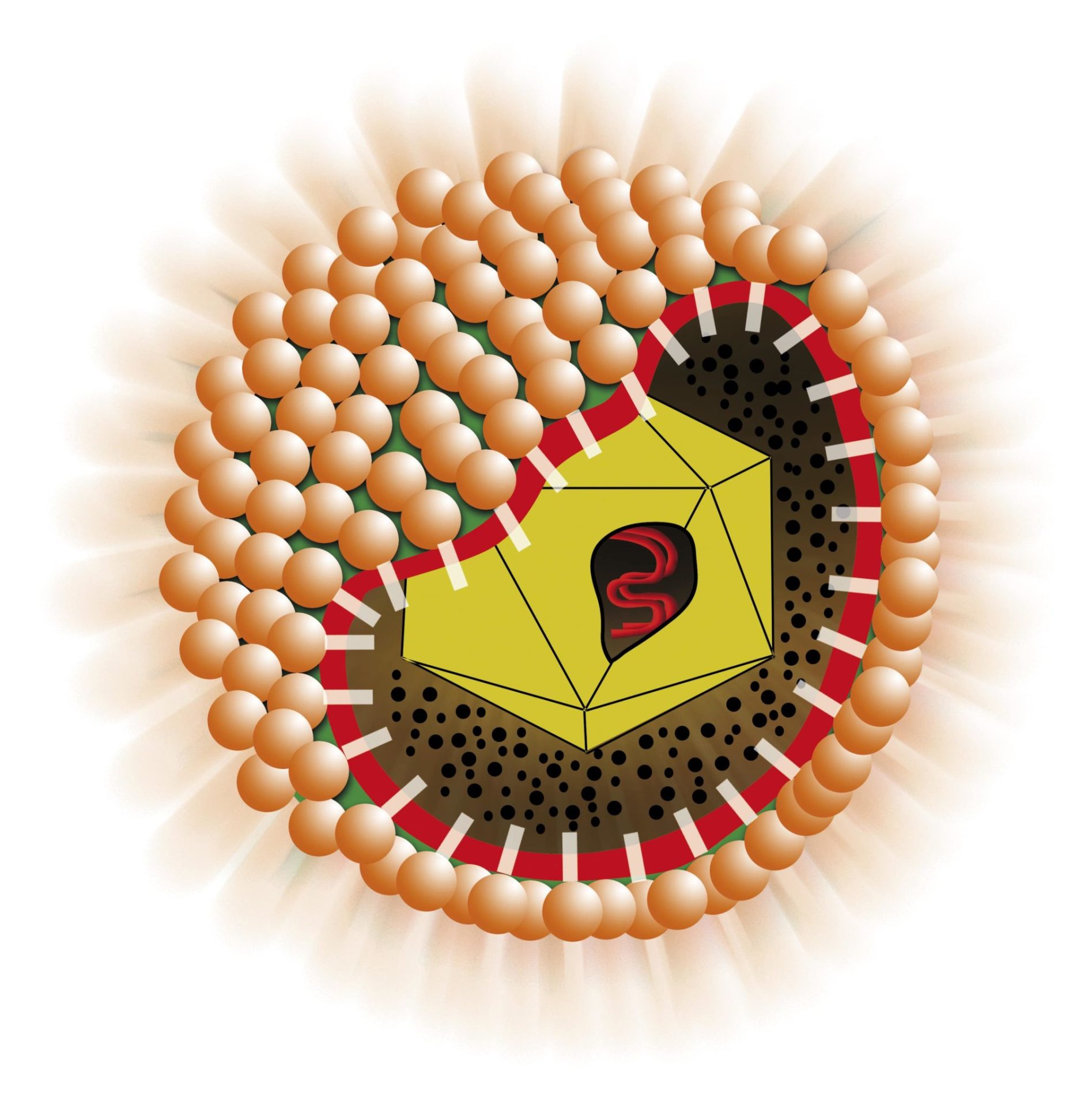
Enzyme ImmunoAssay (ELISA) for the determination of IgM class antibodies to Hepatitis A Virus in human plasma and sera with the “capture” system. The kit may be used for the identification of the viral agent causing hepatitis in the patient and the follow up of the acute phase of the infection.
For “in vitro” diagnostic use only.
The assay is based on the principle of “IgM capture” where IgM class antibodies in the sample are first captured by the solid phase coated with anti hIgM antibody.
After washing out all the other components of the sample and in particular IgG antibodies, the specific IgM captured on the solid phase are detected by the addition of a purified preparation of inactivated HAV, labelled with an antibody conjugated with peroxidase (HRP). After incubation, microwells are washed to remove unbound conjugate and then the chromogen/substrate is added. In the presence of peroxidase the colorless substrate is hydrolysed to a colored end-product, whose optical density may be detected and is proportional to the amount of antibodies to HAV present in the sample.
AVAB.CE: HAV Ab – ELISA
Competitive Enzyme ImmunoAssay (ELISA) for the determination of antibodies to Hepatitis A Virus in human plasma and sera. The kit is used for the follow-up of patients infected by HAV.
For “in vitro” diagnostic use only.
The assay is based on the principle of competition where the antibodies in the sample compete with an anti-HAV specific antibody, labeled with HRP, for a fixed amount of antigen on the solid phase. A purified and inactivated HAV is coated to the microwells. The patient’s serum/plasma is added to the microwell and antibodies to HAV are captured by the solid phase. After washing, the enzyme conjugate is added and binds to the free HAV antigen, if still present. The plate is washed to remove unbound conjugate and then the chromogen/substrate is added. In the presence of peroxidase the colorless substrate is hydrolysed to a coloured end-product, whose optical density may be detected and is inversely proportional to the amount of antibodies to HAV present in the sample. An additive is added to the sample directly into the well to block interferences able to mask the presence of antibodies, mostly appearing in the follow up of vaccination.